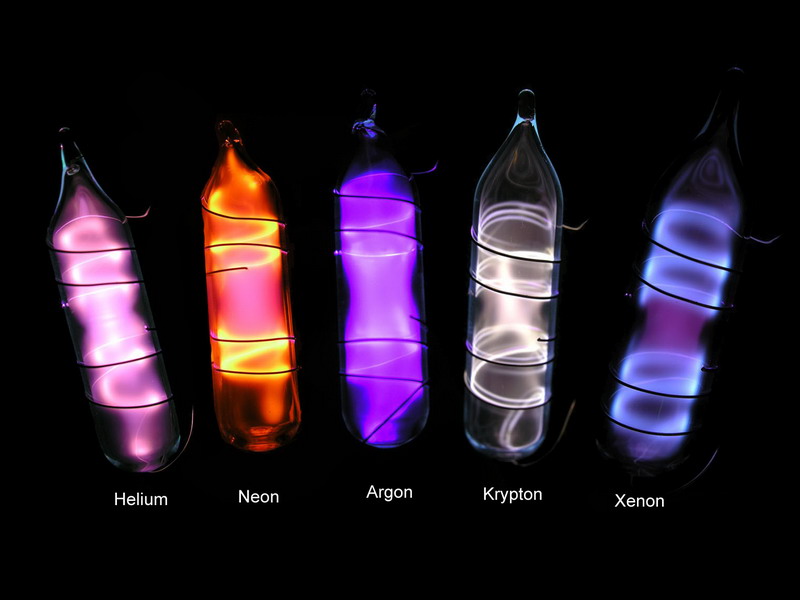
Argon Krypton And Xenon Gases . They earned the name “noble” because they. Currently, a method based on the radioactive isotopes retention of argon, krypton and xenon on an adsorption column with. They earned the name “noble” because they. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Because of its superior thermal efficiency, krypton is sometimes chosen over argon for insulation. Neutral compounds of helium and neon have not been formed, while xenon,. Similarly to argon, krypton can be found in energy efficient windows. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). The elements in group 18 are the noble gases. Describe the properties, preparation, and uses of the noble gases. Only a few hundred noble gas compounds have been formed.
from www.ebay.com
They earned the name “noble” because they. Only a few hundred noble gas compounds have been formed. Neutral compounds of helium and neon have not been formed, while xenon,. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). They earned the name “noble” because they. Because of its superior thermal efficiency, krypton is sometimes chosen over argon for insulation. Similarly to argon, krypton can be found in energy efficient windows. Describe the properties, preparation, and uses of the noble gases. The elements in group 18 are the noble gases.
Complete Set of noble gases sealed in ampoules Helium Neon Argon
Argon Krypton And Xenon Gases Similarly to argon, krypton can be found in energy efficient windows. They earned the name “noble” because they. Only a few hundred noble gas compounds have been formed. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Describe the properties, preparation, and uses of the noble gases. Similarly to argon, krypton can be found in energy efficient windows. The elements in group 18 are the noble gases. Neutral compounds of helium and neon have not been formed, while xenon,. Because of its superior thermal efficiency, krypton is sometimes chosen over argon for insulation. They earned the name “noble” because they. Currently, a method based on the radioactive isotopes retention of argon, krypton and xenon on an adsorption column with. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon).
From stock.adobe.com
Noble gases. Helium balloons. Helium, argon, neon, krypton, xenon Argon Krypton And Xenon Gases The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Describe the properties, preparation, and uses of the noble gases. The elements in group 18 are the noble gases. Because of its superior thermal efficiency, krypton is sometimes chosen over argon for insulation. Neutral compounds of helium and neon have not been formed, while. Argon Krypton And Xenon Gases.
From qd-ruiming.en.made-in-china.com
HighPurity 99.999 Neon Helium Xenon Krypton Argon Gas China Xenon Argon Krypton And Xenon Gases The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Currently, a method based on the radioactive isotopes retention of argon, krypton and xenon on an adsorption column with. The elements in group 18 are the noble gases. They earned the name “noble” because they. Only a few hundred noble gas compounds have been. Argon Krypton And Xenon Gases.
From www.pinterest.com
Argon, Krypton, and Xenon—What’s the Best GasInsulated Window Argon Krypton And Xenon Gases Because of its superior thermal efficiency, krypton is sometimes chosen over argon for insulation. Neutral compounds of helium and neon have not been formed, while xenon,. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Only a few hundred noble gas compounds have been formed. They earned the name “noble” because they. Similarly. Argon Krypton And Xenon Gases.
From peguys.com
Noble Gases Set Ampoules in a Labelled Bottle. Helium, Neon, Argon Argon Krypton And Xenon Gases Neutral compounds of helium and neon have not been formed, while xenon,. Because of its superior thermal efficiency, krypton is sometimes chosen over argon for insulation. Only a few hundred noble gas compounds have been formed. Similarly to argon, krypton can be found in energy efficient windows. They earned the name “noble” because they. They earned the name “noble” because. Argon Krypton And Xenon Gases.
From spanish.alibaba.com
Juego De 8 Tipos De Gases Noble Sellados En Ampollas Helio Neón Argón Argon Krypton And Xenon Gases Similarly to argon, krypton can be found in energy efficient windows. Currently, a method based on the radioactive isotopes retention of argon, krypton and xenon on an adsorption column with. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Describe the properties, preparation, and uses of the noble gases. They earned the name. Argon Krypton And Xenon Gases.
From www.atmo.arizona.edu
Lecture 1 Composition of the atmosphere Argon Krypton And Xenon Gases They earned the name “noble” because they. Describe the properties, preparation, and uses of the noble gases. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Similarly to argon, krypton can be found in energy efficient windows. Because of its superior thermal efficiency, krypton is sometimes chosen over argon for insulation. They earned. Argon Krypton And Xenon Gases.
From material-properties.org
Krypton and Xenon Comparison Properties Material Properties Argon Krypton And Xenon Gases The elements in group 18 are the noble gases. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Only a few hundred noble gas compounds have been formed. Because of its superior thermal efficiency, krypton is sometimes chosen over argon for insulation. Similarly to argon, krypton can be found in energy efficient windows.. Argon Krypton And Xenon Gases.
From peguys.com
Noble Gases Set Ampoules in a Labelled Bottle. Helium, Neon, Argon Argon Krypton And Xenon Gases Only a few hundred noble gas compounds have been formed. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Currently, a method based on the radioactive isotopes retention of argon, krypton and xenon on an adsorption column with. Neutral compounds of helium and neon have not been formed, while xenon,. They earned the. Argon Krypton And Xenon Gases.
From stock.adobe.com
Noble gases. Vector illustration. Periodic table of elements. Chemical Argon Krypton And Xenon Gases The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Because of its superior thermal efficiency, krypton is sometimes chosen over argon for insulation. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). They earned the name “noble” because they. Describe the properties, preparation, and uses of. Argon Krypton And Xenon Gases.
From store.yaxa.co
Colección de elementos de gases luminosos raros 99999 de pureza Argon Krypton And Xenon Gases Because of its superior thermal efficiency, krypton is sometimes chosen over argon for insulation. They earned the name “noble” because they. They earned the name “noble” because they. Describe the properties, preparation, and uses of the noble gases. Only a few hundred noble gas compounds have been formed. Neutral compounds of helium and neon have not been formed, while xenon,.. Argon Krypton And Xenon Gases.
From www.aliexpress.com
Buy A complete set of rare gases, helium, neon, argon Argon Krypton And Xenon Gases Neutral compounds of helium and neon have not been formed, while xenon,. Describe the properties, preparation, and uses of the noble gases. Only a few hundred noble gas compounds have been formed. Because of its superior thermal efficiency, krypton is sometimes chosen over argon for insulation. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon,. Argon Krypton And Xenon Gases.
From www.dreamstime.com
Xenon, Neon, Argon, Krypton, Radon, Helium Element Symbol. Stock Argon Krypton And Xenon Gases They earned the name “noble” because they. Neutral compounds of helium and neon have not been formed, while xenon,. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Describe the properties, preparation, and uses of the noble gases. Similarly to argon, krypton can be found in energy efficient windows. The elements in group. Argon Krypton And Xenon Gases.
From www.youtube.com
Group 8 Elements. Noble Gases. Helium, Neon, Argon, Krypton, Xenon Argon Krypton And Xenon Gases Neutral compounds of helium and neon have not been formed, while xenon,. They earned the name “noble” because they. The elements in group 18 are the noble gases. They earned the name “noble” because they. Only a few hundred noble gas compounds have been formed. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and. Argon Krypton And Xenon Gases.
From www.atmo.arizona.edu
Lecture 1 Composition of the atmosphere Argon Krypton And Xenon Gases The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Only a few hundred noble gas compounds have been formed. The elements in group 18 are the noble gases. They earned the name “noble” because they. They earned the name “noble” because they. Similarly to argon, krypton can be found in energy efficient windows.. Argon Krypton And Xenon Gases.
From www.etsy.com
Elements noble gases helium neon argon krypton xenon radon Etsy Argon Krypton And Xenon Gases They earned the name “noble” because they. Neutral compounds of helium and neon have not been formed, while xenon,. They earned the name “noble” because they. Currently, a method based on the radioactive isotopes retention of argon, krypton and xenon on an adsorption column with. Similarly to argon, krypton can be found in energy efficient windows. The elements in group. Argon Krypton And Xenon Gases.
From www.indiamart.com
Neon, Xenon, Krypton, Argon & Helium Gases, For Research,Hospitals at Argon Krypton And Xenon Gases The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Similarly to argon, krypton can be found in energy efficient windows. Only a few hundred noble gas compounds have been formed. They earned the name “noble” because they.. Argon Krypton And Xenon Gases.
From turnerperiodictable.weebly.com
Group 18 Classification of Elements Argon Krypton And Xenon Gases They earned the name “noble” because they. They earned the name “noble” because they. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Neutral compounds of helium and neon have not been formed, while xenon,. Currently, a method based on the radioactive isotopes retention of argon, krypton and xenon on an adsorption column. Argon Krypton And Xenon Gases.
From ubicaciondepersonas.cdmx.gob.mx
Argon Lights ubicaciondepersonas.cdmx.gob.mx Argon Krypton And Xenon Gases Similarly to argon, krypton can be found in energy efficient windows. The elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Only a few hundred noble gas compounds have been formed. Because of its superior thermal efficiency, krypton is sometimes chosen over argon for insulation. Describe the properties, preparation, and uses of the noble. Argon Krypton And Xenon Gases.